About
Safe and compliant
disposal of quarantine and high-risk biological waste

Many of the infection control, environmental and regulatory risks associated with handling healthcare biosecurity waste can be mitigated with the right management process. That’s why Cleanaway provides specialised collection, containment and disposal services designed for the unique demands of clinical biosecurity.
From hospital quarantine waste and international patient care, to infectious materials linked to notifiable diseases, we deliver integrated solutions that protect people and the environment from exposure to this biohazardous waste. All services are compliant with the Biosecurity Act 2015, relevant EPA regulations and state-specific quarantine requirements – helping you meet safety, sustainability and compliance obligations in one streamlined process.
Whether you’re in a hospital, laboratory, airport or quarantine facility, our end-to-end solutions are tailored to meet your specific biosecurity needs, backed by our national infrastructure and expertise as an approved waste provider under the Department of Agriculture, Fisheries and Forestry (DAFF).
Quarantine and biosecurity waste
Includes quarantine and imported materials, hospital quarantine waste from designated outbreak facilities, contaminated PPE, packaging from declared biosecurity zones, and animal bedding, manure, or tissues from infected or suspect stock.
High-risk clinical and biohazardous waste
Covers international airline waste, biohazardous food, and laboratory or clinical samples from high-risk organisms requiring specialised handling and disposal.
![]()
Service
Who we support
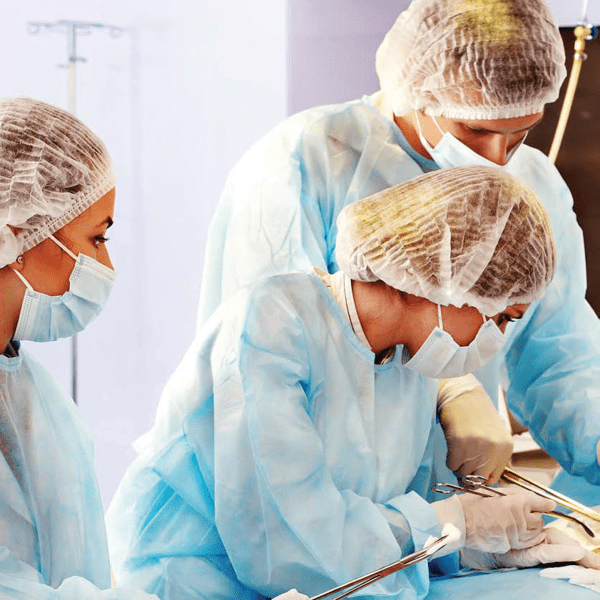
Healthcare providers and laboratories
Hospitals, animal health clinics and labs managing waste from outbreaks, notifiable diseases, infected animals or biohazardous clinical research.

Quarantine and detention facilities
Sites caring for individuals or animals under quarantine control, including immigration detention centres and international quarantine stations.

Ports, airports and border protection agencies
Waste from international cargo, passengers or inspection activities that must be securely contained and destroyed.

Biosecure facilities
Sites operating under strict federal biosecurity licensing, including research facilities and high-level containment laboratories.
![]()
Process
How we manage healthcare biosecurity waste
![]()
Details
Safety and compliance measures
We supply compliant containers to securely collect quarantine and biosecurity waste. All bins are available for temporary or permanent hire.
Collection options include:
- Wheelie bins with lockable lids and red/yellow coding
- Bulk bins and skips that are leak-proof and quarantine marked
- Pallet bins and crates available in custom sizes for dunnage, packaging and residue
- Clinical-grade bags for soft waste in lab settings
![]()
Output
What happens to biosecurity waste
As required by law, biosecurity waste is treated via high-heat incineration at approved facilities to destroy all infectious organisms. This ensures they do not enter the environment, where they could harm the health of animals and humans or destabilise delicate ecosystems.
Our promise
Why choose Cleanaway

A one-stop solution
We offer an expansive range of services, custom waste solutions and expert support to meet the waste management and resource recovery needs of Australian businesses, councils and industries of any size.
Making a sustainable future possible together
We’re leading the way in sustainable waste management and resource recovery, helping Australia to transition to a circular economy and investing in lasting solutions for the benefit of all Australians – now and into the future.

Health, safety and environmental focus
We implement rigorous controls to safely collect, treat and dispose of waste, minimising the risk of harm to people and the environment while meeting strict compliance standards.
Market-leading infrastructure
Our EPA-licensed, state-of-the-art facilities enable safe, efficient waste processing and resource recovery at scale.

